A research group led by Prof. Katsuhiko Mikoshiba from Shanghai Institute for Advanced Immunochemical Studies (SIAIS) at ShanghaiTech University proposes a novel and simple method for creating a stable cytoplasmic antibody, termed STAND, which can be correctly folded and stably expressed in the cytoplasm of the cells. Without the need for complicated amino acid substitutions, it successfully converts aggregation-prone antibodies to various STANDs that are fully functional in vivo.
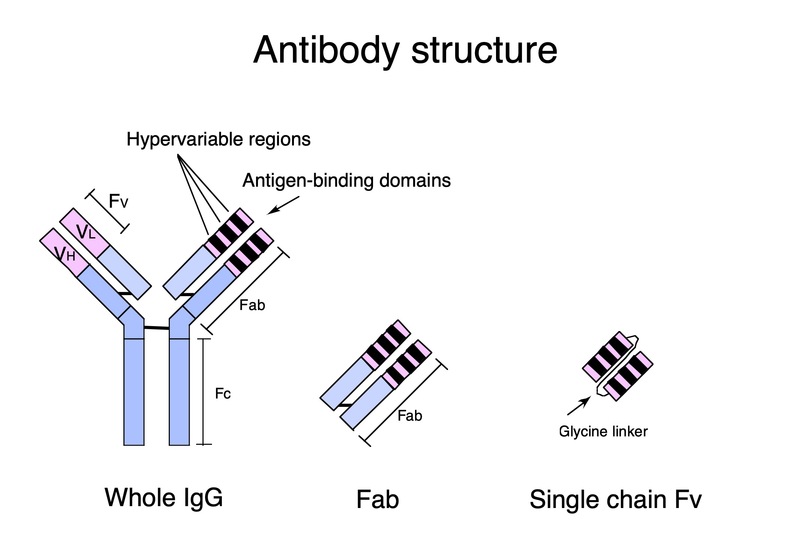
Antibodies are powerful tools in basic research and proven candidates for therapeutic development because they specifically bind to antigens and interfere with targeted molecular pathways by inhibiting protein-protein interactions. While many key drug targets are intracellular molecules, most antibody-based therapies are limited to extracellular targets. Cytoplasmic expression of single-chain Fv (scFv), a most popular format of antibody genes, has been a long-standing challenge due to the tendency to misfold and aggregate; the cytoplasm is a reducing environment, where disulfide bridge formation within the Fvs of the light and heavy chains of scFv molecules can be prevented, thereby causing intracellular aggregation (Fig. 1). Currently, there is no reliable method to engineer stable cytoplasmic intrabodies in mammalian cells.
Here, the research group found a statistically significant negative correlation between aggregation and the net negative charge at pH 6.6, but not at pH 7.4 (Fig. 2a); we also found a statistically significant positive correlation between their pI value and aggregation rate (Fig. 2b). These results indicate that the low pI and strong net negative charge of the scFv antibodies at pH 6.6, but not at pH 7.4, are critical parameters for the stability of cytoplasmic antibodies. Fusion of the peptide tags with the strong net negative charge at pH 6.6 converts aggregation-prone scFvs to STANDs. The STAND for synaptotagmin, a Ca2+ sensor for neurotransmitter release, can be stably expressed in the cytoplasm of dopaminergic neurons (Fig. 3) and inhibit striatal dopamine release, thereby causing motor skill learning (Fig. 4). They also successfully convert aggregation-prone anti-Kras scFv to a STAND, which inhibits in vivo cancer proliferation by mutated Kras—long recognized as an “undruggable” oncogenic protein. (Fig.5). The STAND method shows promise for targeting endogenous cytoplasmic proteins in basic biology and for developing future disease treatments.
This is a collaborative work with Hiroyuki Kabayama from Nippon Veterinary and Life Science University, Professor Shin-ichi Muramatsu from Jichi Medical University, Professor Mitsunori Fukuda from Tohoku University. The researchers published an article entitled An ultra-stable cytoplasmic antibody engineered for in vivo applications in Nature Communications.
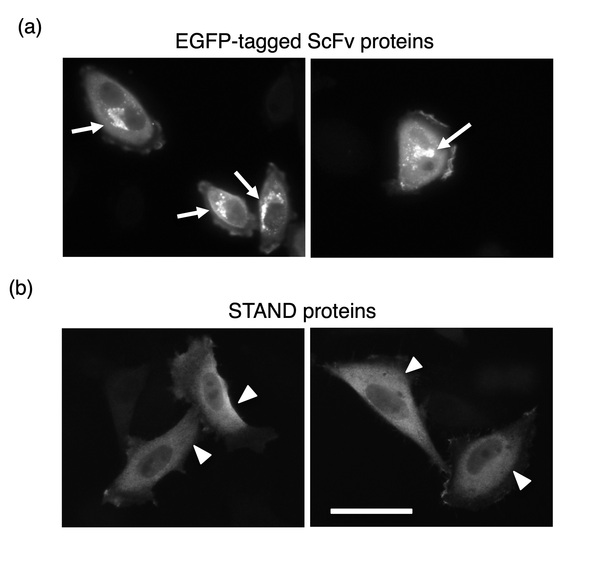
Fig. 1. (a) Cytoplasmic aggregates of scFvs at cytoplasm of mammalian cells. Arrows indicate intracellular aggregates of scFv fused with EGFP tags
(b) Stable expression of STAND proteins
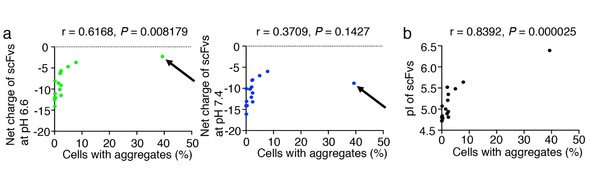
Fig. 2. (a) Statistical correlation between the net negative charge at pH 6.6, not at pH 7.4 of scFvs and aggregates in HeLa cells. The net negative charge of EGFP-tagged scFv-A36 clone (arrows) at pH 7.4 (right panel) was strongly decreased at pH 6.6 (left panel) (b) Statistical correlation between pI value of scFvs and aggregates in HeLa cells
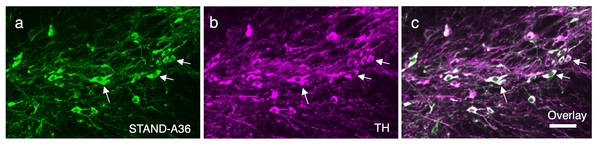
Fig. 3. AAV-mediated stable expression of STANDs in dopaminergic neurons in substantia nigra compacta. STAND-A36: a STAND protein specifically binding to synaptotagmin I/II. Without STANDs, antibody shows aggregation in the cell
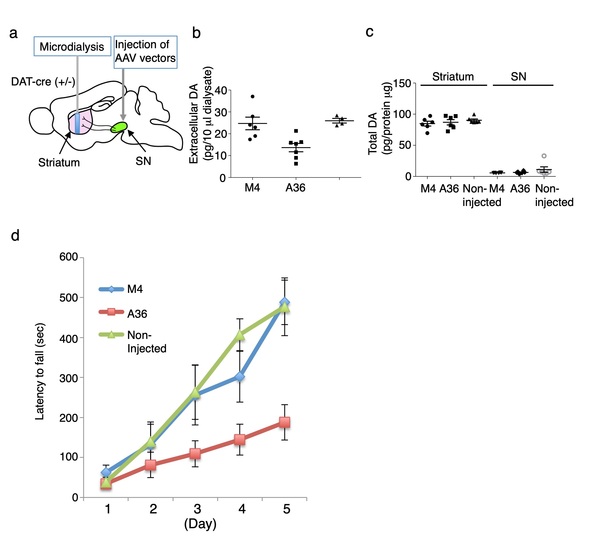
Fig. 4. The STAND-A36 for synaptotagmin inhibits striatal dopamine release and motor skill learning
(a) Extracellular dopamine (DA) was collected by microdialysis. Extracellular (b) and total (c) dopamine in striatum (d) Rotarod test. STAND-M4: a mutant STAND derived from STAND-A36 lacking binding activity to synaptotagmin. Intracellular expression of STAND-A36 decreases basal dopamine release in the striatum in vivo. A microdialysis was performed in the striatum of the right cerebral hemisphere of mice 33 days after AAV injection into the SNc
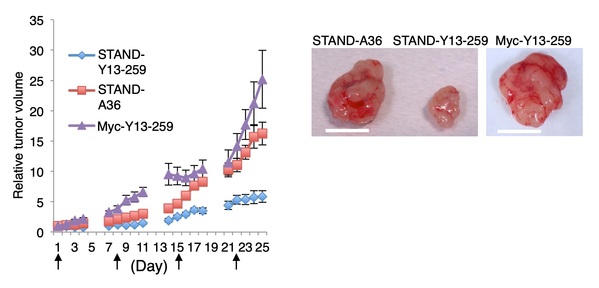
Fig. 5. The STAND-Y13-259 for Kras inhibits cancer proliferation driven by mutated Kras. Lenti-viral vectors expressing scFv proteins indicated were intratumoral injected every week. STAND-Y13-259; a STAND protein specifically binding to Kras was successfully made from an aggregation-prone scFv-Y13-259 clone
Read the article: https://doi.org/10.1038/s41467-019-13654-9



