Published on Oct 20, 2015 in the Proceedings of the National Academy of Sciences with the title of “Agonist antibody that induces human malignant cells to kill one another”, the Lerner lab in SIAIS ShanghaiTech University finds a totally new approach to Leukemia treatment. Prof. Richard Lerner and Prof. Kyungmoo Yeafrom SIAIS ShanghaiTech University, recently tested 20 of receptor-activating antibodies previously discoveredin the lab, against acute myeloid leukemia cells from human patients and found an antibody against thrombopoietin (TPO) receptor which canconvertacute myeloid leukemia cells into natural killer (NK) cells specifically and efficiently.
The team in SIAIS ShanghaiTech University, previously reported a phenomenon called receptor pleiotropism in which agonist antibodies against known receptors induce cell fates that are very different from those induced by the natural agonist to the same receptor.The thrombopoietin (TPO) receptor is well-surface expressed at a high percentage in acute myeloid leukemia cells. Natural thrombopoietin cannot convert AML cells from patients. However, in their latest study, using an agonist antibody against TPOR, specific and efficient conversion of AML cells from AML patients into NK cells was achieved. It should be noted that the antibody has no effect on differentiation of normal bone marrow cells. The induced AML cells have extensive filopodia at their surface and express the CD11c dendritic cell marker. Moreover, these induced cells synthesize large amounts of markers of NK cells involved in the mechanism of killing, such as perforin, granzyme B, and IFN- γ. Specific killing of the target AML cells is mediated by multiple fine filopodia from the dendritic extensions of the induced killer AML cells. RNAseq data further confirmed the up-regulation of dendritic cellmarkers such as CD80, CD83, CD86, CD123, CCR7 andmany NK markers of the death receptor pathway such as celldeath surface receptor (FAS), FAS ligand, and tumor necrosisfactor (TNF- α), after TPOR antibody stimulation.
Surprisingly, the induced AML cells’ cancer-killing effect appeared to be purely fratricidal. The researchers found that unrelated breast cancer cells did not die off in large numbers when in the presence of the NK cells. Such fratricidal therapies provide advantages over conventional cancer treatment in many aspects, such as reduced adverse side effects and total elimination of cancer-cell population instead of reduction, etc.“We’re in discussions with pharmaceutical companies to take this straight into humans after the appropriate preclinical toxicity studies,” Prof. Richard Lernersummarized.
Prof. Kyungmoo Yea (first author) and Prof. Richard Lerner (corresponding author) are affiliated with SIAIS,ShanghaiTech University.
http://www.pnas.org/content/early/2015/10/20/1519079112.abstract
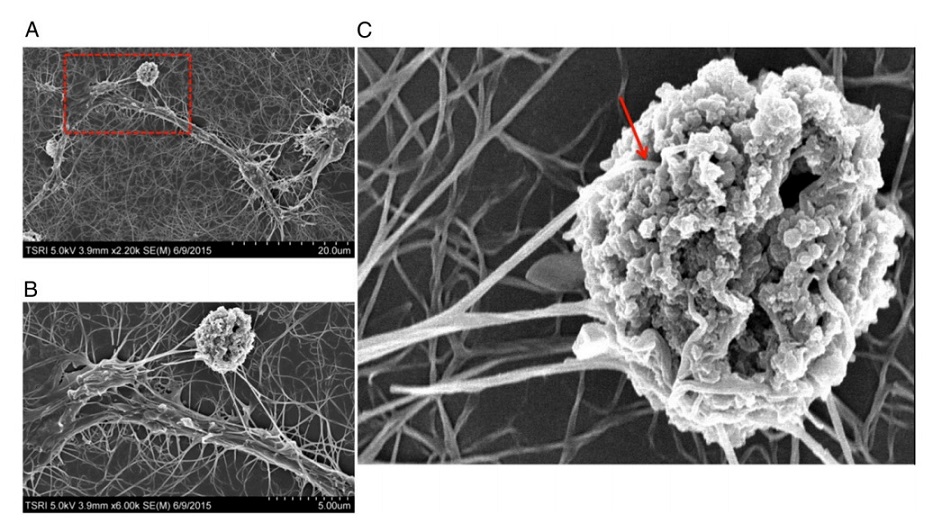
Multiple fine filopodiafrom the dendritic extensions of theinduced NK cells attaching to the target AML cells.



