Recently,Research Associate Professor Xuekai Zhu from T Cell Engineering Laboratory atSIAIS, and Professor Xingxu Huang from SLST have developed a new technology forthe preparation of genome-edited chimeric antigen receptor (CAR) T cells basedon non-viral vectors. The researchers used the CRISPR/Cas9 system to achieveefficient knockout of the PD-1 gene in human T cells, while stably integratingand expressing CD133-specific CAR via the transposon system. The study waspublished in the journal Human GeneTherapy titled with Nucleofection with Plasmid DNA forCRISPR/Cas9-Mediated Inactivation of Programmed Cell Death Protein 1 in CD133-SpecificCAR T Cells.
Usingviral vector-transduced T cells is the mainstream direction of CAR-T celltherapy. However, the production and quality control of clinical grade viralvectors is very expensive. Thereare currently only a few units in the world that are qualified and capable ofproviding clinical grade CAR virus vectors. In addition, CRISPR/Cas9-mediatedgenome editing in CAR T cells requires the presence of Cas9. In order to avoidthe immunogenicity of non-human Cas9 protein in the edited T cells, it iscommon to use electroporation to ensure that Cas9 is transiently present. TCell Engineering Laboratory has long been working on methods for deliveringgenes into T cells using non-viral vectors. In this study, CRISPR/Cas9 was usedin combination with the transposon system, and a one-step plasmid-only methodwas used to achieve gene editing and CAR expression simultaneously in T cells. Theresearchers further confirmed that the technology can prepare genome-edited CART cells, in which CAR expression and gene editing efficiency can meet clinicalneeds (Fig.1). Advantages of the non-viral plasmid vector for delivering genesrelative to viral vector transduction include cost reductions to 1/10 of thelatter and larger capacity for delivered genes.
Inrecent years, the success of immunotherapy represented by CAR T cells andPD-1/PD-L1 blocking antibodies has made cancer treatment a new era. The 2018Nobel Prize in Physiology or Medicine was awarded to scientists who madeimportant contributions to the development of the immune checkpoint blockadetherapy, including CTLA-4 and PD-1 antibodies. The combination of CAR T cellsand immune checkpoint inhibition is expected to counter the immunosuppressiveenvironment of solid tumors and reduce the systemic immune-related side effectscaused by checkpoint blocking antibodies. It has been a research hotspot in thefield of tumor immunotherapy. In this study, the researchers successfullyknocked out the PD-1 gene in CD133-CAR T cells using genome-edited CAR T celltechnology. They found that CD133-CAR T cells with PD-1 gene silence were morepotent in killing tumor cells in vitro than traditional CAR T cells. And significantly prolonged survival oftumor-bearing mice and inhibition of tumor growth were shown for PD-1KO CAR Tcells in animal experiments (Fig.2).
Infuture research, T Cell Engineering lab will use this technology to studyunknown regulatory factors that affect T cell function, and develop powerfulCAR T cells or universal CAR T cells for solving problems such as the treatmentof refractory solid tumors and difficult large-scale preparation of autologousCAR T cells.
Graduatestudent Bian Hu from Xingxu Huang’s group is the first author. Prof. Xuekai Zhuand Prof. Xingxu Huang are co-corresponding authors.
Link tofull paper: https://www.liebertpub.com/doi/full/10.1089/hum.2017.234
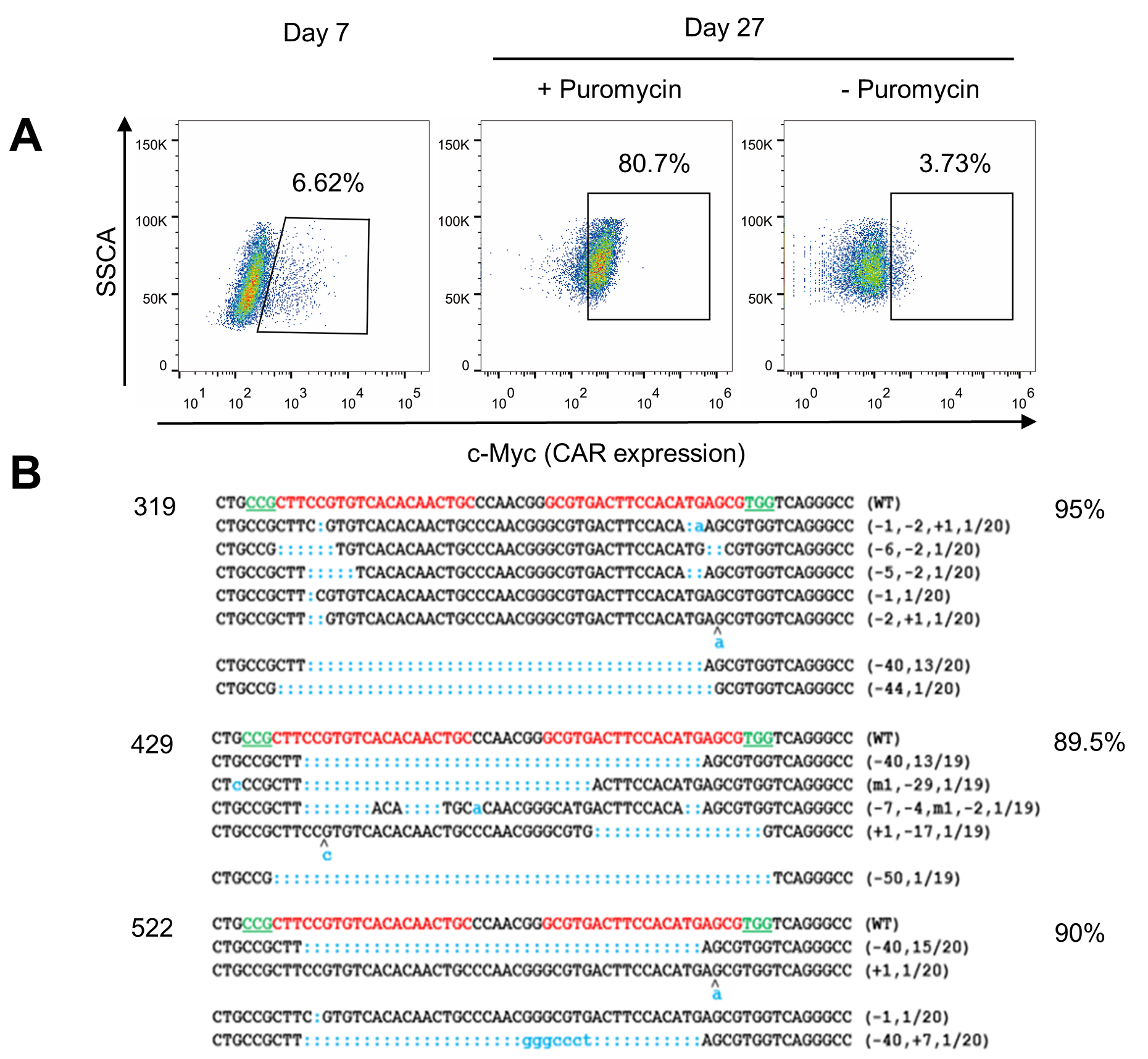
Fig.1: Achievehigh expression of CD133-CAR and high efficiency of PD-1 gene knockout simultaneouslyin human T cells via non-viral vectors

Fig.2: PD-1knockout CD133-CAR T cells show stronger anti-tumor function



