On October 25, 2018, the Science published online the researcharticle from the team led by ShanghaiTech Distinguished Professor-in-Residence Rao Zihe with the title “An electrontransfer path connects subunits of a mycobacterial respiratory supercomplex”.
On the strength of cryo-electronmicroscopy (cryo-EM) with high resolution (3.5 Å) structure of mycobacterialrespiratory supercomplex, the team revealed a new electron transfer mechanism couplingquinone oxidation with oxygen reduction. Furthermore, superoxide dismutase (SOD) was discovered,for the first time, directly involved in the assembly of the supercomplex and workin concert. These new findings have laid a solid groundwork for the R&D ofnew drugs against tuberculosis that pose grave threats on human health.
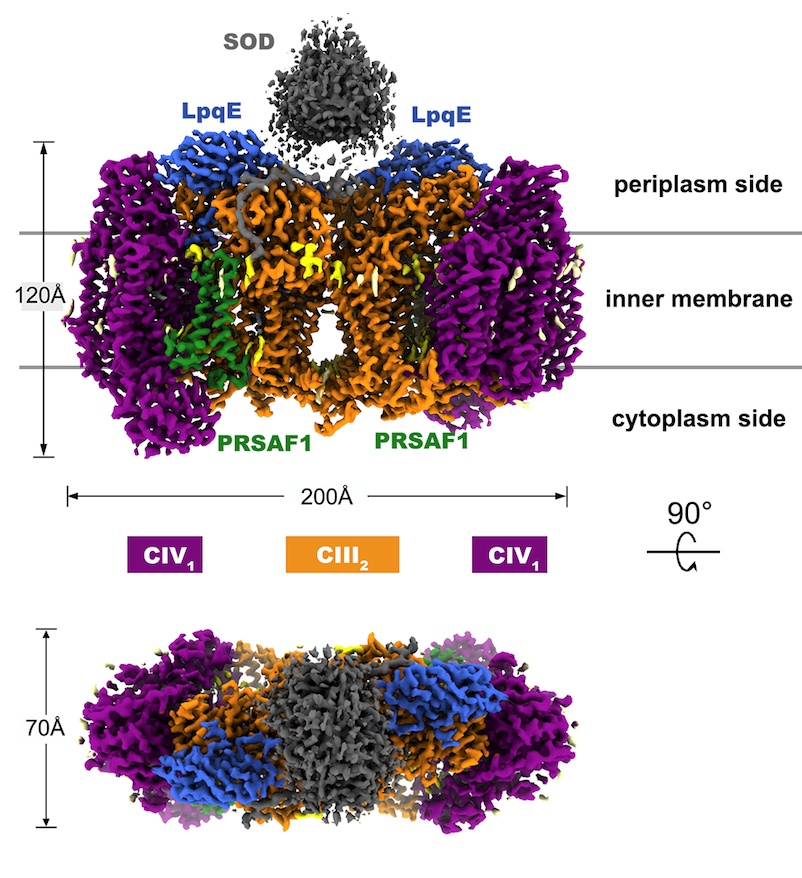
The 3.5-Å resolution cryo-EM structure of mycobacterialrespiratory supercomplex CIII2CIV2SOD2
Respiration is one of the mostbasic energy metabolisms in life, and helps energetic transform from thesubstances (e.g. sugar, amino acid and fatty acid) into the adenosinetriphosphate (ATP) that could be immediately consumed by the life body. It is run mainly by five largetransmembrane complexes on the microbial cytoplasmic membrane or the innermitochondrial membrane: complexI (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (quinone:cytochrome c oxidordeuctase), complexIV (cytochrome c oxidase) and complexV (ATP synthase), together with two carriers for electron transfer, quinone andcytochrome c, which therefore iscalled the respiratory chain. Among them, electron transfers inside and betweenthe complexes I-IV through cascading with redox reaction, thus forming theelectron transfer chain, coupling which the transmembrane proton gradient iscreated for driving the synthesis of ATP by complex V. Previous studies haveshown that the components of respiratory chain could be further assembled intosupercomplexes to promote tandem reaction among them. This is of greatsignificance in the regulation of energy metabolic efficiency and variousphysiological processes. The disorder in supercomplex assembly would be closelyrelated to the occurrence of diseases in higher animals; when interrupted in microorganisms,it would be an important strategy for developing new drugs to inhibit their growthand infection.
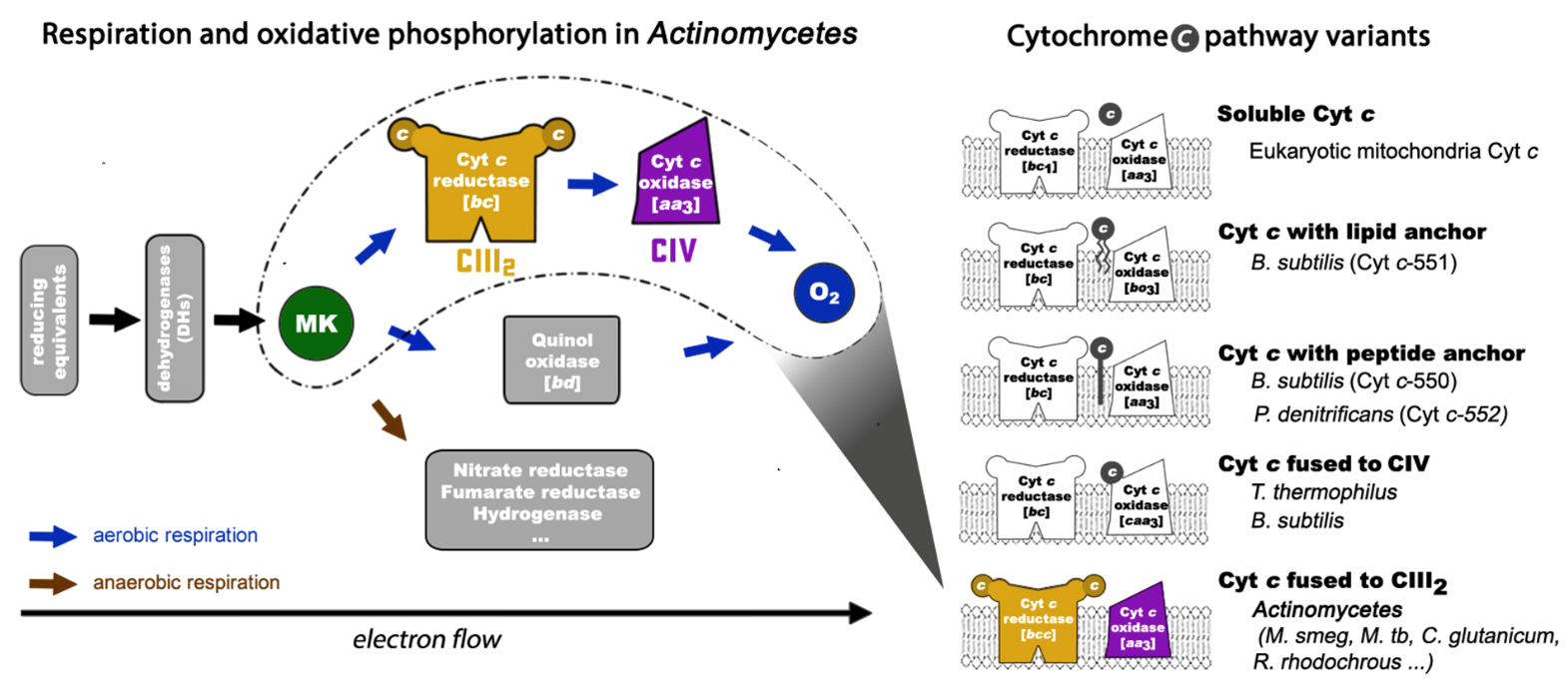
Respiratory chain of Actinobacteria, a phylum containing Mycobacteria
“It is common for theassembly of complex III and complex IV to form a supercomplex, which is particularlyimportant in energy production for Actinobacteria, a phylum containing Mycobacterium tuberculosis and multiple pathogenicbacteria. On the other hand, there were no extensive and direct interactionsbetween complex III and complex IV in the previous reported structuresincluding mammalian respiratory supercomplex I1III2IV1.Meanwhile, the cytochrome c proteinin mitochondria exists in free soluble form to mediate electron transferbetween complex III and complex IV. So, it is still questionable on whether it shuttlesinside the supercomplex or across multiple supercomplexes. In this work, wehave revealed the whole electron transfer path from complex III to complex IVand the tandem reaction mechanism for the two complexes. Such revelation answersone of the widely concerned scientific questions that has long been unresolvedin the respiratory field, following our first report of the crystal structureof the eukaryotic mitochondrial complex II in the Cell in 2005,” said Professor Rao.
As explained by Gong Hongri andXu Ao,doctoral students of the Nankai University and co-first authors of thisarticle, success was the fruit from years of preparation and technicalaccumulation by the team in the study of Mycobacteriumtuberculosis. Their close cooperation runs from the purification andoptimization of samples to activity validation, from preparation of cryo-EM samplesto improvement of data processing methods and eventually to the resolve of near-atomicresolution structural model. Such cooperation coupled with harmonized effortsin multiple biochemical and biophysical methods, such as lipidomics analysis,atomic absorption spectrometry and electron paramagnetic resonance test,empowered the researchers to gain comprehensive intimate knowledge of the wholeelectron transfer path inside this particular supercomplex.
“Another unexpecteddiscovery is superoxide dismutase (SOD). Despite in zymologic function it haslong been regarded correlated with redox reaction of the respiratory chain byradicals scavenging and also with the host’s immunoreactions during microbialinfection, direct evidence is still absent. Additionally, the molecular mechanism of SOD involved insuch connection is rather controversial. From the viewpoint of the structuralbiology for the first time, we confirmed the direct interaction in themycobacterial periplasm between SOD and the respiratory chain complex as wellas SOD’s capability of scavenging the potential free radicals to drive redoxreaction. Moreover, this finding implies an important mechanism of Actinomycetes represented by Mycobacterium tuberculosis to resistimmunoreactions in the host’s macrophage, thus bringing new revelation tofurther understanding of the interactions between Mycobacterium tuberculosis and its host,” said key members ofthe team Dr. Wang Quan and Research Associate Professor Li Jun.
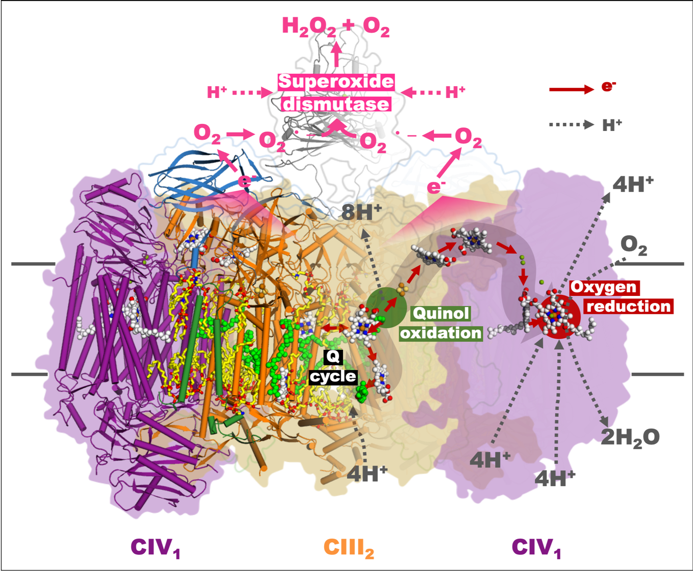
SOD involved in the redox reactioninside mycobacterial respiratory supercomplex
The research bears greatsignificance in the R&D of new drugs. The World Health Organization (WHO) reportsthat tuberculosis has become a leading infectious disease in the world. Fordecades, the long-term use of isoniazid, rifampicin and other drugs hasresulted in increasingly acute drug resistance. The multiple-drug resistancetuberculosis and even extremely drug-resistant tuberculosis have become one ofthe grave challenges in treating tuberculosis. Studies of recent yearsindicated that targeting energy metabolic system can remarkably address thedrug resistance, which has been increasingly attracting attention. In 2012, theFDA in U.S. expedited approval of the Bedaquiline, the first drug treating themultiple-drug resistance tuberculosis and it gained access to China in March2018. The working principle of this drug is to inhibit the respiratory chainsystem from synthesizing ATP to wipe out Mycobacteriumtuberculosis.
“The complex III under ourresearch is a highly popular drug target. The pharmaceutical molecule Telacebec(Q203) in the phase II clinical trials is just suppressing the binding ofnatural substrate of the complex to block the aerobic respiration pathway of Mycobacterium tuberculosis, thus to playits pharmacological role. Our research will serve as a great powerhouse for furtherimprovement of this drug and development of even new drugs with betterefficacy,” highlighted by Professor Rao.

Binding site of substrate(MK) and the potential drug in mycobacterial complex III
The research team led by Professor RaoZihe has long been committed to the research on the structural biology ofemerging and reemerging infectious disease pathogens in China. This article isthe second one published in the Science this year following publication of the assembly mechanism of herpes virusearlier this year. Multiple institutions have been contributed to this researchwith ShanghaiTech University being one of the three key initiators. Research AssociateProfessor Li Jun of the Shanghai Institute for Advanced Immunochemical Studies isthe co-first author of this article and Professor Rao Zihe is theco-corresponding author. Professor Jiang Biao, the doctoral student Wang Shuhuiand Research Associate Professor Yang Xiuna have also contributed to this work.In addition, the National Center for Protein Science (Shanghai) provided someof technical support for this work.
Link: http://science.sciencemag.org/content/early/2018/10/24/science.aat8923



