A team of scientists led by Dr. Yang Bei and Dr. Ian A. Wilson from ShanghaiTech’s SIAIS and Dr. Lu Lu and Dr. Jiang Shibo from Fudan University’s School of Basic Medical Sciences have developed a pan-coronavirus (pan-CoV) fusion inhibitor EK1. EK1 targets the HR1 domains of various human coronavirus (HCoV) spike (S) proteins to prevent the host-HCoV membrane fusion process and inhibits the host entry of HCoVs into the target cells. The same study also demonstrates for the first time that the HR1 region of coronavirus (CoV) S protein is indeed a druggable and conserved target for pan-CoV fusion inhibitors, thereby providing theoretical basis and framework for future development of broad-spectrum anti-HCoV drugs. Their work entitled “A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike” was published online in Science Advances on April 10, 2019.
Continuously emerging highly pathogenic HCoVs remain a significant threat to human health, as illustrated by the life-threatening outbreaks of SARS-CoV and MERS-CoV in this century. To date, there have been no effective vaccines and treatments available for prevention and treatment of HCoV infections. Besides, zoonotic CoVs exhibit high genomic variability and harbor great potential to cross the species barrier and infect humans, posing further challenges for development of broad-spectrum anti-HCoV drugs. Recently, the World Health Organization (WHO) has proposed the concept of defense against “Disease X,” i.e. a human disease with serious international epidemic potential caused by a currently unknown pathogen, including a new CoV. (http://www.who.int/blueprint/priority-diseases/en/). Therefore, the development of a broad-spectrum and highly-efficient HCoV fusion inhibitor holds great significance for the prevention and treatment of infections caused by the current and emerging CoVs. To this end, how to identify a conserved target for development of a broad-spectrum anti-HCoV drug is a key scientific question to be addressed in the field.
In 2014, the research team at Fudan University derived a peptide fusion inhibitor MERS-HR2P from the HR2 region of the MERS-CoV spike protein (Lu L et al., Nat. Commun. 5:3067, 2014). MERS-HR2P competitively bound to the S protein HR1 region and effectively inhibited MERS-CoV infection. Nevertheless, MERS-HR2P failed to cross-inhibit SARS-CoV infection, and vice versa, the peptide derived from SARS-CoV HR2 could not cross-inhibit MERS-CoV infection (Fig. 1). What is more complicated is that the HR1 region of α-HCoVs is 14 amino acids longer than that of β-HCoVs (Fig. 1B), and exhibits different surface electrostatic potential distributions (Yan L et al., Acta Crystallogr D Struct Biol. 74:841-51, 2018). Therefore, whether HR1 is a viable target for pan-CoV inhibitors remains unresolved in the field.
In this study, the joint research team first established cell-cell fusion systems for multiple existing HCoVs. They then performed cross-screening using these systems and finally identified a peptide that has pan-HCoV fusion inhibitory activity. This peptide derived from the HCoV-OC43 HR2 region (OC43-HR2P) was further optimized into peptide EK1 with better solubility and higher inhibitory activity. In both pseudo typed and live HCoVs infection assays, the peptide EK1 exhibited improved potency and breadth in inhibiting infection by multiple α-HCoVs and β-HCoVs (Fig. 1).
To unravel the molecular basis for the pan-CoV inhibitory effect of the EK1 peptide, the joint research team further determined co-crystal structures of EK1 in complex with the HR1 regions from multiple HCoVs. The results clearly show that EK1 can act broadly on the HR1 regions of both α-HCoVs and β-HCoVs through forming extensive and highly-conserved hydrophobic and hydrophilic interactions (Fig. 2), thereby antagonizing the formation of the autologous 3HR1-3HR2 six helical bundle that is pivotal for the entry of HCoVs into the host cells. In summary, the structural study not only revealed that EK1 exhibits good conformational and surface charge plasticity to accommodate variation among the HR1s from different HCoVs, but more importantly explained the conserved molecular basis for the HR1-EK1 interaction, further indicating that HR1 region can indeed serve as a promising target for the development of pan-CoV inhibitors.
Encouraged by these promising results, the joint research team went on to perform in vivo studies in mice. Administration of EK1 via the nasal route demonstrated highly protective effects in both HCoV-OC43 and MERS-CoV infection mouse models. Moreover, EK1 manifests satisfactory safety profiles and low immunogenicity in vivo, suggesting it possesses promising clinical potential to be further developed into a broad-spectrum antiviral agent against current and emerging HCoVs.
This study was supported by grants from the Ministry of Science and Technology to Dr. Lu Lu and Dr. Jiang Shibo, from the National Natural Science Foundation of China to Dr. Lu Lu and Dr. Yang Bei, and start-up support from ShanghaiTech to the Laboratory of Antibody Structure.
Paper Link: https://advances.sciencemag.org/content/5/4/eaav4580

Figure 1. The potent and broad-spectrum antiviral activity of EK1. A. The antiviral mechanism of HR2P peptides. B. The sequences of the designed peptides: HR1Ps, HR2Ps and EK1. C. Inhibitory activity of EK1 in cell-cell fusion mediated by the various CoV S proteins. D. Inhibitory activity of EK1 against pseudo typed CoVs infection. E. Inhibitory activity of EK1 on live HCoV replication for MERS-CoV, HCoV-OC43, HCoV-229E and HCoV-NL63, respectively.
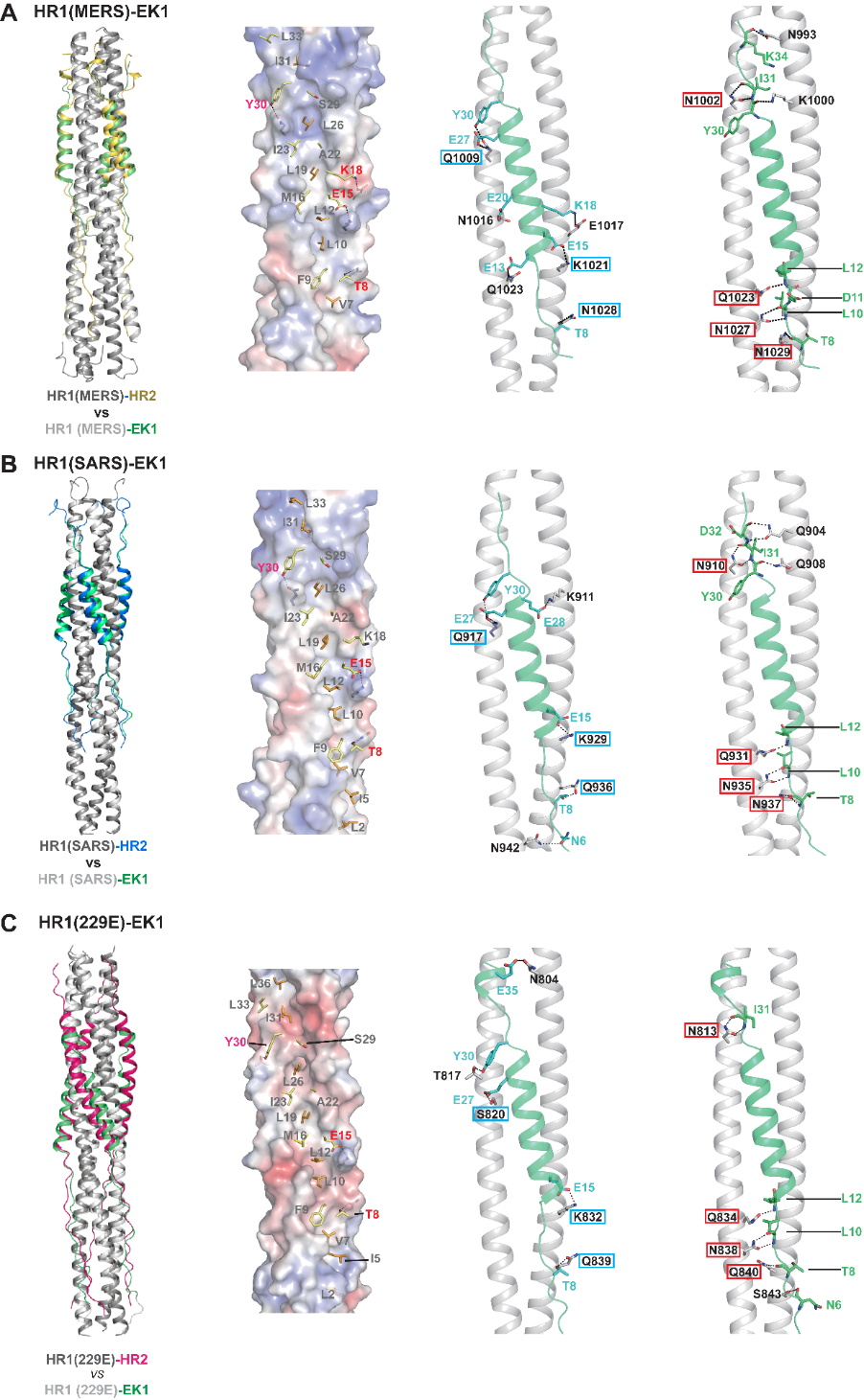
Figure 2. Structural Studies of EK1 in complex with the HR1 regions from MERS-CoV (A), SARS-CoV (B) and 229E (C) unravel the molecular basis for the pan-CoV inhibitory effect of EK1 peptide. 1st panel: Structural comparison of the HR1-EK1 6-HB bundles and cognate HR1-HR2 6-HB bundles reveals that the EK1 peptide binds to the 3HR1 core of different HCoVs in a similar manner to that of the native HR2 of the corresponding HCoV. 2nd-4th panel: the extensive and highly-conserved hydrophobic and hydrophilic interactions between EK1 and different HR1s; EK1 residues that are involved in hydrophobic packing are shown as stick models on the electrostatic surfaces of 3HR1 cores(2nd panel), and HR1 residues from MERS-CoV, SARS-CoV and 229E that meditate highly conserved side chain-to-side chain (3rd panel) and side chain-to-main chain (4th panel) hydrophilic interactions with EK1 residues are boxed in cyan and red respectively.



