On September 30th, 2019, Advanced Science published a collaborative study led by Professor Richard Lerner and Professor Guang Yang from Shanghai Institute for Advanced Immunochemical Studies (SIAIS) of ShanghaiTech University, entitled “DNA-encoded libraries: aryl fluorosulfonates as versatile electrophiles enabling facile On-DNA Suzuki, Sonogashira, and Buchwald reactions”. The study has identified, for the first time, a series of (hetero) aryl fluorosulfonates (ArOFS) as versatile electrophiles for on-DNA cross-coupling reactions.
DNA-encoded combinatorial chemical library (DEL) approach, which combines synthesis, amplification, and sequencing of DNA with DNA-compatible organic reactions, has emerged as a powerful technology in small molecule drug discovery. It enables screening of ultra-large compound libraries in an unprecedented high-throughput and cost-efficient manner. To date, several drug candidates derived from their corresponding DEL hits, such as soluble epoxide hydrolase inhibitor GSK2256294 and death domain receptor-associated adaptor kinase RIP1 inhibitor GSK2982772, have progressed to late-stage clinical trials. Despite these successes, the great potential of DEL technology in drug discovery has yet been fully realized. One of the most fundamental challenges is the synthesis of high-quality libraries with complex structures and spatial diversities, which depends on new, and versatile DNA-compatible chemical reactions.
To develop the next generation diversity-oriented DELs and to realize the full potential of DEL technology, the research teams from SIAIS have established ArOFs as highly efficient electrophiles in palladium-catalyzed on-DNA Suzuki, Sonogashira, and Buchwald cross-coupling reactions. The study has demonstrated that the formation of C(sp2)-C(sp2), C(sp2)-C(sp), and C(sp2)- N bonds in these cross-coupling reactions were facile at low reaction temperature and under air. The reactivity profile of -OFs and other common electrophilic leaving groups (-I > -Br > OFs > Cl) makes it possible to carry out sequential on-DNA synthesis to form poly-substituted aryl and/or heteroaryl molecules in DELs. Moreover, these new on-DNA coupling reactions could also be applied to the late-stage modification of phenol-containing bioactive natural products (NPs), a significant advantage in nDEL library construction (ref. Angew. Chem. Int. Ed. 2019, 58, 9254 – 9261). All of these will greatly facilitate the design and synthesis of diverse DEL libraries.
This work represents a significant advancement in the research of on-DNA chemical reaction methods and a milestone in the field of DEL research. Research Assistant Professor Dr. Hongtao Xu and Postdoc Dr. Fei Ma from SIAIS are the co-first authors of this article; SIAIS Professors Richard Lerner and Guang Yang are the corresponding authors. Dr. Wenzhang Chen and Jiakang Chen at SIAIS analytical chemistry platform carried out MS analyses. ShanghaiTech University is the first affiliated institution of this research. This work was supported by grants from the National Natural Science Foundation of China, and JPB Foundation in US.
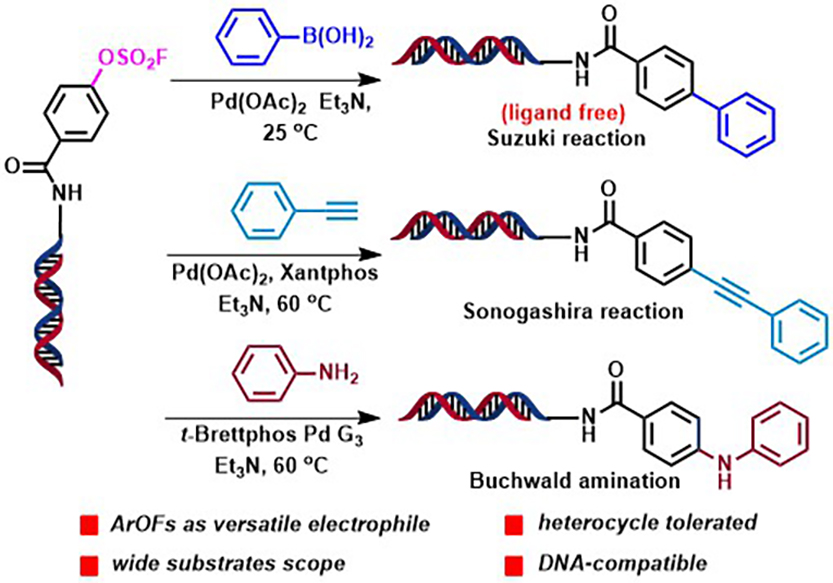
Figure 1. (Hetero)aryl fluorosulfonate as versatile electrophiles enables facile on-DNA cross-coupling reactions
Read the paper at:https://onlinelibrary.wiley.com/doi/10.1002/advs.201901551



