Professor Katsuhiko Mikoshiba and Research Associate Professor Kozo Hamada of Cell Calcium Signaling laboratory at SIAIS published a review paper entitled ‘IP3 Receptor Plasticity Underlying Diverse Functions’ in Annual Review of Physiology in November 2019. The paper reviews recent progress on the IP3 receptor structure and function, and the authors propose a new concept of how protein plasticity within IP3 receptors would enable it to regulate diverse functions at cellular microdomains.
In the past 40 years, from its discovery to recent progress, numerous studies have demonstrated that IP3 receptors mediate diverse functions at cellular microdomains, including organelle-contact sites between mitochondria and endoplasmic reticulum, and its aberrant regulation results in a variety of human diseases, such as spinocerebellar ataxia. Recent cryo-electron microscopy and X-ray crystallography resolved IP3 receptor structures and have begun to integrate with diverse microdomain functions. This review highlights recent research progress on the IP3 receptor structure and function, and the authors provide a novel model of how these structural plasticity mechanisms triggered by dynamic transformations of the IP3 receptor assembly at each microdomain, including Ca2+-dependent regulation, binding proteins, and post translational modifications, are involvedin state-dependent properties of locally diverse functions.
Drs. Mikoshiba and Hamada joined to SIAIS of the ShanghaiTech University in April 2019, after conducting calcium signaling researches at the University of Tokyo and RIKEN inJapan for 20 years. Their research focuses on the cellular and molecular mechanism of calcium signaling in the normal and disease states.
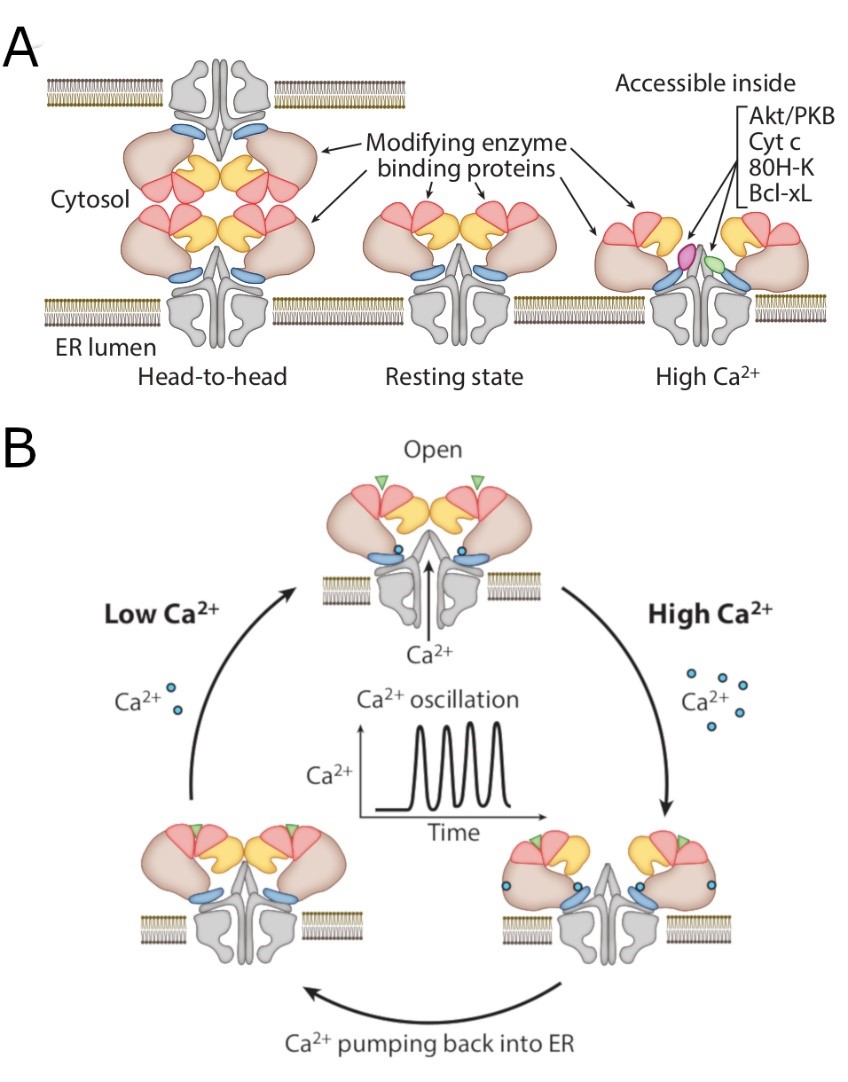
(A) Amodel for transformations of the IP3 receptor. On the left is ahead-to-head arrangement of tetrameric IP3 receptor. Ca2+-dependent structural changes that should enable binding proteins and/or modifying enzymesto access inside IP3 receptors.
(B) Ca2+ waves or oscillation should determine structural plasticity of IP3 receptors at each microdomain.
Read more at:https://www.annualreviews.org/doi/abs/10.1146/annurev-physiol-021119-034433



