At 2:00 AM (Beijing Time) on 24th April, a research article titled “Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol” as reported by the joint research team led by Prof. Rao Zihe, Distinguished Adjunct Professor at Shanghai Institute for Advanced Immunochemical Studies (SIAIS), was published via First Release in Science. This work, for the first time, successfully determined the three-dimensional structures of drug-target complexes of the mycobacterial arabinosyltransferases EmbA-EmbB and EmbC-EmbC (EmbC2). High-resolution images of these structures revealed the precise molecular mechanism by which ethambutol, the first-line anti-TB drug, targets these Emb proteins, thereby providing a fundamental structural basis for the development of new anti-TB drugs to treat resistance.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is the leading cause of human fatalities due to any infectious disease worldwide. First-line anti-TB drugs in current clinical therapeutics have been discovered throughout more than half a century. Of great concern, however, is the emergence of multi-drug-resistant (MDR) and extensively drug-resistant (XDR) TB, which has further exacerbated the global burden of TB and at the same time continues to lead to a reduction in clinical recovery rates. Thus, there is an urgent need to understand the drug targets of Mtb and to develop new anti-TB drugs.
Mtb has a complex cell wall compared to Gram-negative and Gram-positive bacteria. Its core structure, mycolyl-arabinogalactan-peptidoglycan (mAGP), is composed of three highly unusual elements covalently linked together: (i) long-chain mycolic acids (MA), (ii) a highly branched arabinogalactan (AG), and (iii) a cross-linked network of peptidoglycan (PG) and other key components such as lipoarabinomannan (LAM). The mycobacterial cell wall acts as a natural barrier surrounding the cell membrane. Inhibition of Mtb cell wall assembly is an established strategy for anti-TB chemotherapy, and the targeting of cell-wall biosynthesis is regarded as the mode of action of first-line drugs like ethambutol and isoniazide. The membrane-embedded Emb proteins, EmbA, EmbB and EmbC, which are involved in AG (EmbA/EmbB) and LAM (EmbC) biosynthesis are regarded as the targets of ethambutol. However, the molecular mechanism of ethambutol inhibition remains unclear, thus hindering the design of new-generation ethambutol-based drugs aimed at conquering drug resistance.
Prof. Rao Zihe’s joint team has made a long-term commitment to studying the structures of key Mtb drug targets and anti-TB drug development. Structures of respiratory chain super-complex CIII2CIV2SOD2 (targeted by Q203, an anti-TB drug in Phase II clinical trials) and MmpL3 (targeted by SQ109, an anti-TB drug in Phase II clinical trials) in complex with a series of drug candidates have been reported by the joint team and published in Science in 2018 and in Cell in 2019 respectively. On 24th April another significant breakthrough was reported by the team: they have determined the three-dimensional structures of EmbA-EmbB and EmbC-EmbC in complex with drug ethambutol. These structures reveal the precise molecular mechanism by which ethambutol targets these Emb proteins.
This discovery represents the culmination of more than six years arduous work by the team during which time they overcame numerous difficulties including non-expression of target proteins, poor diffraction of the crystals, phase problems, unavailability of the natural substrates and lack of assay activity. Ultimately they determined the structures of substrate/drug bound Emb complexes using both X-ray crystallography and cryo-EM single particle analysis techniques. These data have helped researchers finally solve the fifty-year puzzle of ethambutol inhibition mechanism.
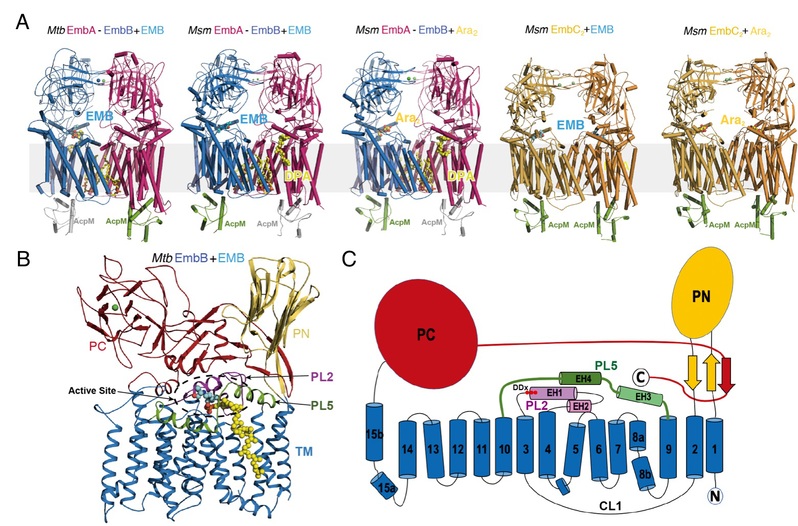
Figure Legend: (A) Structures of Mycobacterium tuberculosis (Mtb) and Mycobacterium smegmatis (Msm) EmbA-EmbB-AcpM2 and EmbC2-AcpM2 in complex with substrates (Ara2, DPA) and drug ethambutol (EMB); (B) Domains and structural features of Emb proteins, represented by Mtb EmbB; (C) Topological diagram of Emb proteins
These structural and functional studies have revealed that EmbA-EmbB form a functional heterodimeric complex, whilst EmbC acts as a symmetric homodimer. An unexpected discovery is the association of acyl-carrier-protein (AcpM) with the Emb proteins on the cytoplasmic surface, thus together forming EmbA-EmbB-AcpM2 and EmbC2-AcpM2 complexes. Each Emb protein contains common features including a 15-helix TM domain and two jelly-roll fold containing periplasmic domains, with the active site located in a pocket at the junction between the TM domain and the periplasmic domains. Substrate-bound structures have shown how the arabinosyl donor (DPA) and acceptor di-arabinose (Ara2) bind within the active site. Ethambutol-bound structures have further demonstrated that the drug also binds to the same sites (D-site and A0-site) as both substrates close to the catalytic aspartic acid residue in EmbB and EmbC, thus inhibiting AG and LAM. Therefore, the majority of drug-resistant mutations are located nearby to the ethambutol-binding site of EmbB and EmbC. Direct or indirect effects would occur upon mutation, leading to a lower binding affinity of ethambutol. Consequently, avoiding these mutation effects should be taken into consideration in new generation drug design in order to solve the resistance problem. Thus, the above findings provide a fundamental basis for development of improved ethambutol-based anti-TB drugs targeting Emb proteins.
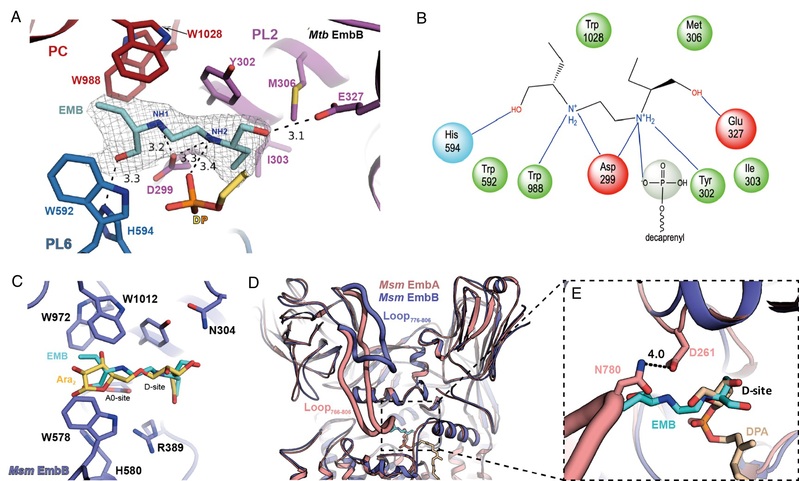
Figure Legend: (A) Structural details of ethambutol binding; (B) Schematic diagram of ethambutol binding; (C) Structural superposition shows ethambutol overlaps with the substrate binding sites (D-site and A0-site for arabinose); (D) Structural superposition shows ethambutol overlaps with the arabinose group of DPA; (E) Zoom-in view of superposition in (D)
This work marks a comprehensive achievement in the anti-TB drug target research area led by Prof. Rao’s joint team. Currently, the team is taking full advantage of the Zhangjiang Biomedical Industry Base (Shanghai) to push forward new anti-TB drug development and to accelerate transformation from research results into productive forces through comprehensive cooperation. PhD student Zhao Yao from ShanghaiTech University and Dr. Zhang Lu from Nankai University are the co-first authors of the paper. Prof. Rao Zihe from SIAIS of ShanghaiTech University, Prof. Gurdyal S. Besra from Birmingham University, UK, Research Associate Prof. Li Jun from SIAIS of ShanghaiTech University, and Principal Investigator and Assistant Prof. Wang Quan from SIAIS and the School of Life Science and Technology at ShanghaiTech University are co-corresponding authors. The paper is firstly affiliated with ShanghaiTech University; X-ray and cryo-EM data collections were assisted by Shanghai Synchrotron Radiation Facility (China) and Bio-Electron Microscopy Facility of ShanghaiTech University.

Group photo of the research team members (from the left to right):
Prof. Li Jun, Dr. Zhao Yao, Prof. Rao Zihe, Dr. Zhang Lu, Prof. Wang Quan
Link to the article:
https://science.sciencemag.org/content/early/2020/04/22/science.aba9102



