Professor Katsuhiko Mikoshiba from SIAIS, with the French Professor Valentin Nägerl’s group unraveled the morphological mystery of astrocytes. Misa Arizono, a former PhD student in Mikoshiba’s lab is the first author of this report in Nature Communications 2020.
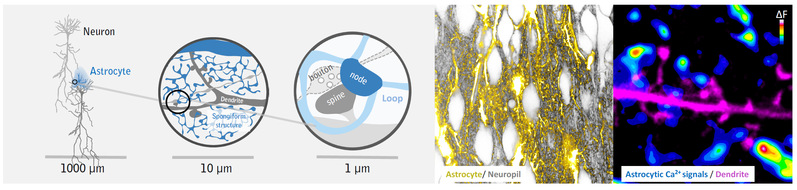
Astrocytes, which are the most numerous glial cells in the brain, use Ca2+ signals to regulate their biochemical activity and communication with other brain cells. While single astrocytes are in principle well positioned to influence thousands of neuronal synapses that lie within their anatomical domain with great dexterity, astrocytic Ca2+ signals have long been thought to be too sluggish and sprawling for mediating any type of fast and specific actions. However, this view has come from low-resolution studies that have looked at the soma and major branches of the astrocytes. More recent work on the tiny but relevant astrocytic processes that actually contact synapses suggests that the situation may be similar to neurons, where fast and local synaptic Ca2+ signals mediate high-speed point-to-point communication. However, the anatomical basis of such specific signaling by astrocytes has remained unclear, owing to difficulties in resolving the complex morphology of astrocytes (and synapses), while also recording their Ca2+ activity in live brain tissue. To overcome this problem, we turned to 3D-STED microscopy, which offers a much higher spatial resolution than regular light microscopy and can reveal the morphology of astrocytes and neurons in great detail. By combining it with confocal Ca2+ imaging to monitor Ca2+ activity, and FRAP experiments to assess biophysical properties, we could elucidate the anatomical basis of Ca2+ signals in astrocytes.
We observed that astrocytic processes form a reticular meshwork of nodes and shafts that formed ring-like structures. The nodes exhibited spontaneous Ca2+ signals that stayed local most of the time, but could also spread to neighboring nodes via the shafts. FRAP experiments established that the astrocytic node/shaft structure generally supports compartmentalized signaling, yet also permits signal propagation across multiple nodes. Mapping the Ca2+ data onto the STED images of the morphology, showed that the majority of astrocytic Ca2+ signals were associated with single synapses suggesting that astrocytes are capable of engaging in synapse-specific communication.
Altogether, our study shines new light on the nanoscale organization of astrocytes in live brain tissue (organotypic and acute brain slices and in vivo), identifying astrocytic nodes as the elusive anatomical structure that may regulate neuronal communication at single ‘tripartite synapses’.
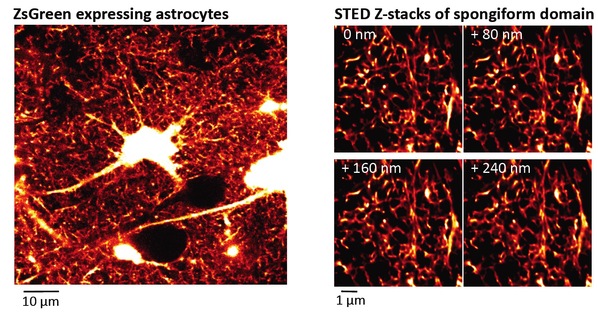
Figure 1. Spongiform domain of astrocyte revealed by live STED microscopy
Left: Confocal overview image of astrocytes expressing ZsGreen
Right: Z-stack STED images of spongiform domain showing an elaborate reticular meshwork
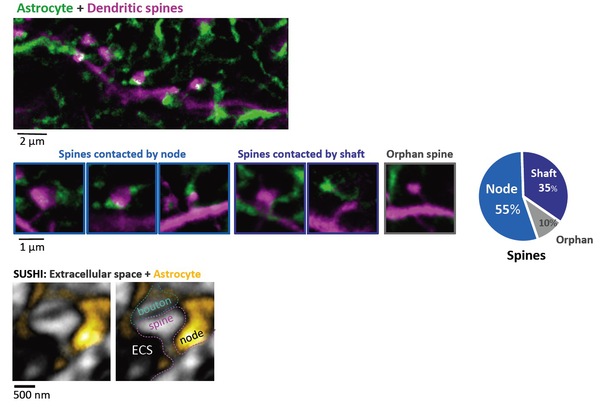
Figure 2. Majority of dendritic spines are contacted by 'astrocytic nodes’
Top: Two-color STED image of morphological interaction between astrocytic spongiform domain (green) and a dendrite (magenta).
Middle: Images (left) and percentage (right) of spines contacted by nodes, shafts, and spines lacking an astrocytic structure in their vicinity.
Bottom: Image of extracellular space (black) surrounding a synapse (gray) and astrocyte (yellow).
Right image indicates the putative identity of the synaptic components.
Reference: Structural basis of astrocytic Ca2+ signals at tripartite synapses Nature Communications volume 11, Article number: 1906 (2020) https://www.nature.com/articles/s41467-020-15648-4
Misa Arizono’s website : https://arizono0202.wixsite.com/misa-arizono