On October 30, 2020, a research article entitled “Structural basis of trehalose recycling by the ABC transporter LpqY-SugABC” was published in Science Advances by a joint research team led by Prof. Rao Zihe, a Distinguished Adjunct Professor at Shanghai Institute for Advanced Immunochemical Studies (SIAIS). This work reported four high-resolution cryo-electron microscopy structures of the mycobacterial LpqY-SugABC complex to reveal how it binds and passes trehalose through the membrane to the cytoplasm. These findings enrich the understanding of how ABC transporters facilitate substrate transport across the membrane in Gram-positive bacteria.

It is well known that ABC transporters are ubiquitously found in prokaryotes and eukaryotes and play vital roles in transporting a diverse range of substrates across the cell membrane. While many have been characterized from Gram-negative bacteria, very little is known about the structure and mechanism of transport for their Gram-positive counterparts.
Here, the research team reports four high-resolution cryo-EM structures of Mycobacterium smegmatis LpqY-SugABC, a trehalose-specific importer, to reveal how trehalose passes through the membrane to the cytoplasm. As such, it is the first time that the mechanism of an ABC transporter has been revealed in Gram-positive bacteria. It is also the first time that a complete complex of a lipid-modified substrate binding protein and an ABC transporter have been determined. A unique feature of this system is the initial mode of capture of the trehalose at the LpqY interface. Uptake is achieved by a pivotal rotation of LpqY relative to SugABC, moving from an open and accessible conformation to a clamped conformation upon trehalose binding. In the resting state, the orientation of LpqY (SBP) relative to SugABC is significantly different by comparison to the equivalent substrate binding proteins in the E. coli (a Gram-negative bacteria) maltose transporter MBP-MalFGK2 and Archaeoglobus fulgidus molybdate transporter ModB2C2A. According to these differences, the research team suggests that LpqY binds to the transporter prior to substrate binding and is held there throughout the entire process of import. This type of association in the resting state of ABC transporters has not previously been observed. The research team suggests this type of arrangement could occur in other ABC transporters where lipid-modified substrate-binding proteins are involved.
In mycobacteria (including Mtb), trehalose is an integral component of various glycolipids that are important cell wall structures and thus vital for the survival and virulence of these bacteria. Notably, trehalose analogues have been shown to have great potential as antibacterial drug leads. To date, LpqY-SugABC has been identified as the only trehalose importer in these bacteria and is required for these trehalose analogues to be transported across the membrane. So, these structural data presented here will greatly facilitate the design and development of new trehalose analogs as antibacterial drugs. Furthermore, the outbreaks of hypervirulent strains of Clostridium difficile are associated with trehalose metabolism. These strains can metabolize low concentrations of the trehalose, which contributes to hypervirulence of the pathogen and increases the risk of disease. This research offers some insights into finding the analogues of trehalose with anti-bacteria activities, which may help to reduce prevalence. Clostridium difficile disease.
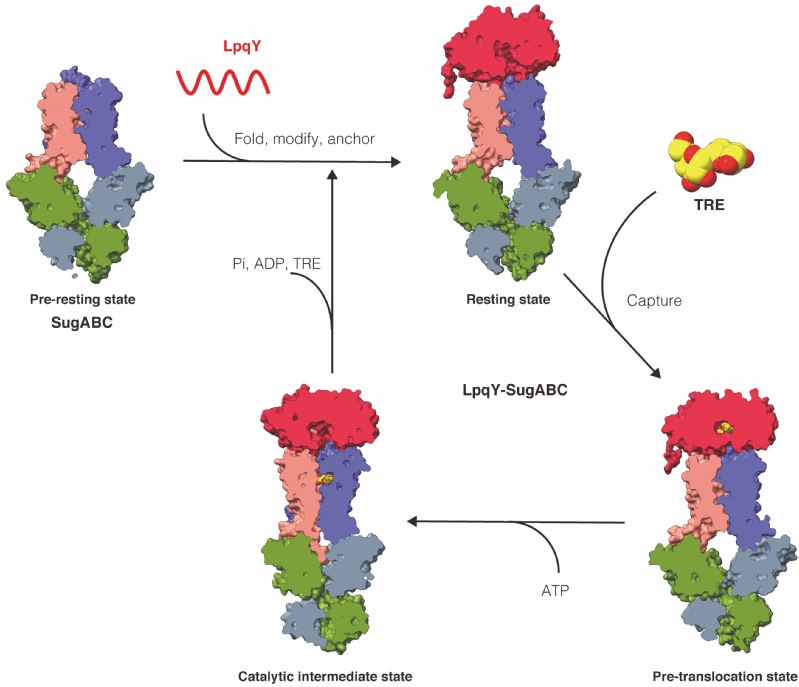
Mechanism of trehalose transportation across the cell membrane
This paper, the culmination of work which has been ongoing for several years, represents a comprehensive achievement in the anti-TB drug target research work of the joint team led by Prof. Rao. Ph.D. student Liu Fengjiang from ShanghaiTech University, Liang Jingxi from Nankai University and Dr. Gao Yan from Tsinghua University are the co-first authors of the paper. Prof. Rao Zihe and Research Associate Prof. Zhang Bing from SIAIS of ShanghaiTech University are the co-corresponding authors. The paper is firstly affiliated with ShanghaiTech University. Cryo-EM data collections were assisted by the Bio-Electron Microscopy Facility of ShanghaiTech University.
Link for the article:
https://advances.sciencemag.org/content/advances/6/44/eabb9833.full.pdf



