On July 7, a research team led by Research Associate Professor Zhang Heqiao and Distinguished Adjunct Professor Roger Kornberg (Lab of Structural Biochemistry) at Shanghai Institute for Advanced Immunochemical Studies (SIAIS), ShanghaiTech University, published a research article in Cell. The study, titled “Structures of the measles virus polymerase complex with non-nucleoside inhibitors and mechanism of inhibition,” elucidates the molecular mechanism by which non-nucleoside inhibitors suppress the RNA polymerase complexes of measles virus (MeV) and Nipah virus (NiV).

The measles virus is a highly contagious non-segmented, negative-sense RNA virus (nsNSV) with a basic reproduction number (R0) ranging from 12 to 18. Measles, the disease caused by this virus, typically presents with symptoms such as fever, cough, conjunctivitis, and a distinctive rash. The World Health Organization (WHO) reports that there were an estimated nine million measles cases in 2021, over 10.5 million in 2022, and more than 10.3 million in 2023 globally. In 2022 and 2023, over 136,000 and 107,500 measles-related deaths were reported, respectively. Despite the availability of a safe and affordable vaccine, up to 5% of individuals fail to generate sufficient protective antibodies following the recommended two-dose regimen. Given its high transmissibility and the large number of susceptible individuals, the measles virus remains an ongoing global health threat. A few non-nucleoside inhibitors, such as ERDRP-0519, AS-136A, and GHP-88309, have been reported to inhibit the activity of the MeV polymerase. Unlike nucleoside analog drugs that competitively bind to the nucleotide-binding site, the mechanisms of action of these non-nucleoside inhibitors are not yet fully understood.
Nipah virus, which also belongs to the Paramyxoviridae family of nsNSVs, has caused several outbreaks in Asia in recent years. Nipah virus can be transmitted from animals to humans or between humans, leading to severe symptoms such as encephalitis and pneumonia, with mortality rates as high as 70%. Currently, vaccines and antiviral drugs targeting Nipah virus remain under development, and no licensed products are available for prevention or treatment. Measles and Nipah viruses encode eight and six proteins in their respective genomes, with the RNA-dependent RNA polymerase complex (L-P complex), composed of the L and P proteins, being an attractive target for antiviral drug development.
The team has long focused on the regulation of eukaryotic and viral gene transcription. In December 2024, they reported the structural features of the NiV RNA polymerase complex in Science Advances, laying an important foundation for the present study. In their latest work, the team first resolved the apo-state structure of the MeV L-P complex at 3.0 Å resolution using single-particle cryo-electron microscopy (cryo-EM). The structure showed that the L protein assembles with a tetrameric P protein to form a conical-shaped polymerase complex (Figure 1A). Subsequently, the team determined cryo-EM structures of the MeV L-P complex bound to the non-nucleoside inhibitors ERDRP-0519 (Figure 1B) and AS-136A (Figure 1C) at 3.4 Å and 3.3 Å resolution, respectively. Interestingly, both inhibitors bind to a pocket near the catalytically essential “GDN” loop. Structural comparisons revealed that these non-nucleoside inhibitors employ an allosteric inhibition mechanism to inhibit polymerase activity by inducing a conformational change in the GDN loop—from a “GDN-in” to a “GDN-out” state (Figure 1D). This conformational rearrangement likely disrupts the coordination of magnesium ions and nucleotides, thereby inhibiting RNA synthesis. The newly identified inhibitor-binding pocket holds promise as a novel druggable site for developing therapeutic agents against measles virus.
By comparing the newly-determined structure of the MeV L-P complex bound to ERDRP-0519 with the team’s previously reported apo structure of the NiV L-P complex, the researchers hypothesized that ERDRP-0519 might also bind to and inhibit NiV polymerase. Using surface plasmon resonance (SPR) and in vitro biochemical assays, they confirmed the binding and inhibitory activity of ERDRP-0519 against the NiV polymerase. Finally, they resolved the cryo-EM structure of the NiV L-P complex bound to ERDRP-0519 at 3.0 Å resolution, which revealed that, as with the MeV, the inhibitor binds to the pocket near the GDN loop (Figure 1E) and induces a similar conformational rearrangement from “GDN-in” to “GDN-out,” leading to polymerase inactivation. This study provides a critical structural basis for the rational design of antiviral drugs targeting measles and Nipah viruses.
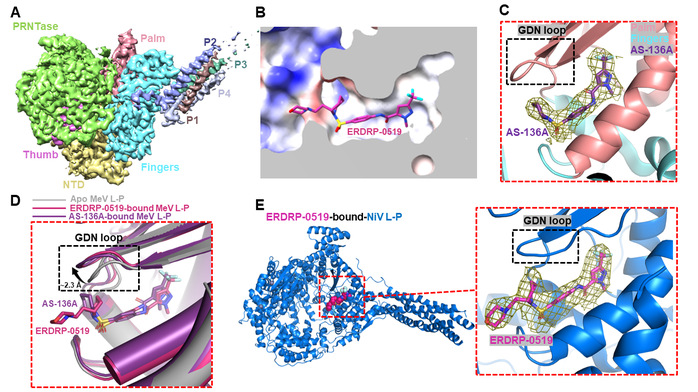
Figure 1. Molecular mechanism of non-nucleoside inhibitors blocking the polymerase activity of measles and Nipah viruses
(A) Cryo-EM reconstruction of the measles virus L-P complex.
(B) Molecular details of ERDRP-0519 binding to the measles virus L-P complex.
(C) Molecular details of AS-136A binding to the measles virus L-P complex.
(D) Conformational change in the measles polymerase induced by non-nucleoside inhibitors.
(E) Molecular details of ERDRP-0519 binding to the Nipah virus L-P complex.
Wang Yiru, a 2024 PhD graduate and Senior Engineer Zhao Lixia from SIAIS are the co-first authors. Zhang Heqiao and Prof. Roger Kornberg are the co-corresponding authors. ShanghaiTech University is the first affiliation of the paper.
*This article is provided by Zhang Heqiao



